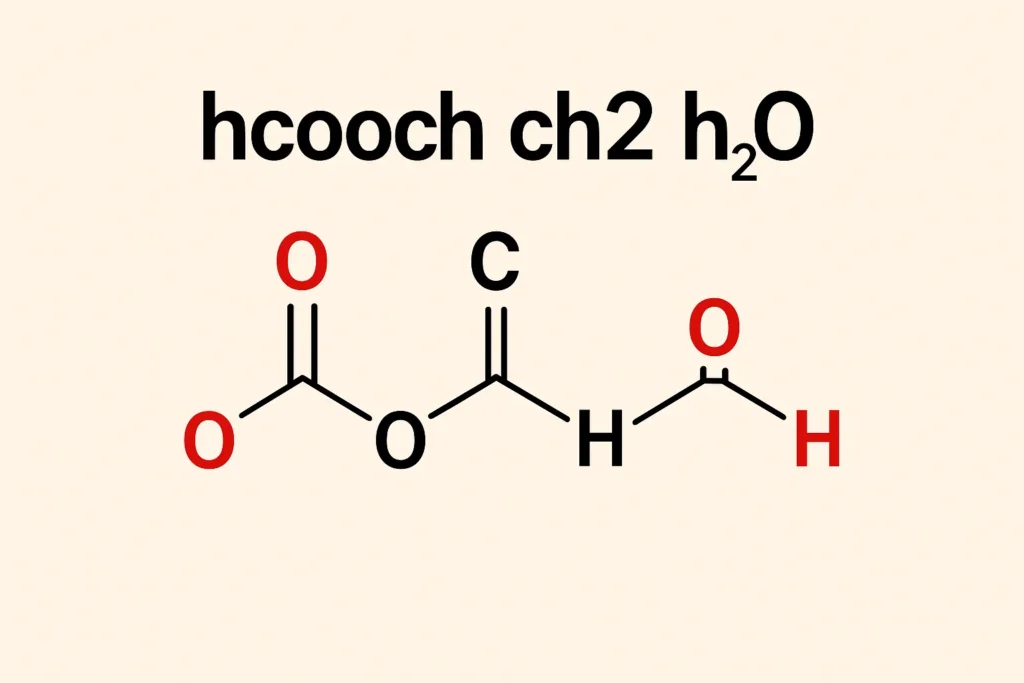
Chemistry often involves understanding how different molecules interact, transform, and contribute to broader industrial and biological processes. Among important small molecules, methyl formate (HCOOCH₃), methylene (CH₂), and water (H₂O) hold significant relevance in organic synthesis, atmospheric chemistry, and biochemical reactions. This article explores their structures, properties, and roles in chemical processes.
Methyl formate is the simplest ester of formic acid (HCOOH) and methanol (CH₃OH).
Its structure contains:
- A formyl group (HCO–)
- Linked through an oxygen atom to a methyl group (–CH₃)
| Property | Description |
|---|---|
| Physical State | Colorless liquid |
| Odor | Ether-like fragrance |
| Boiling Point | ~31–32°C |
| Solubility | Slightly soluble in water; miscible with organic solvents |
- Industrial solvent for resins and adhesives
- Intermediate in the production of formamide
- Used in fragrances and flavorings
- Employed in fuel cell research
Methyl formate undergoes:
- Hydrolysis → formic acid + methanol
- Reduction → methanol or formaldehyde derivatives
- Nucleophilic attack on the carbonyl carbon
Methylene is not a stable, isolated molecule under standard conditions. It is a carbene, meaning it has a divalent carbon atom with two nonbonded electrons, making it extremely reactive.
- Singlet methylene (¹CH₂): Highly reactive
- Triplet methylene (³CH₂): Most stable form
Methylene is generated in:
- Photolysis or pyrolysis of diazomethane
- Reactions involving halomethanes (e.g., CH₂Cl₂)
- Combustion processes
Methylene readily inserts into:
- C–H bonds
- O–H bonds
- Double bonds, forming cyclopropane derivatives
It plays a central role in organic synthesis, atmospheric reactions, and radical-based mechanisms.
Water is one of the most abundant and chemically essential compounds on Earth. Its unique molecular structure gives it exceptional properties.
- Polar molecule due to bent structure and electronegativity difference
- High heat capacity
- Excellent solvent for ionic and polar compounds
- Hydrogen bonding enables cohesion, adhesion, and surface tension
Water participates in:
- Hydrolysis reactions
- Acid–base chemistry
- Redox processes
- Biological reactions such as metabolism
While these three compounds are not typically grouped in a single classic reaction, they can be conceptually connected:
In the presence of water:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
A generated CH₂ carbene can insert into C–H or O–H bonds of many molecules, but direct reactions with methyl formate or water are less common and highly conditions-dependent.
- Methyl formate (HCOOCH₃) is a valuable industrial ester with important uses in synthesis and solvents.
- Methylene (CH₂) is a reactive intermediate crucial in organic chemistry, especially carbene-based reactions.
- Water (H₂O) remains a universal solvent and a central participant in hydrolysis and biochemical reactions.
Together, these molecules illustrate the diversity and interconnectedness of chemical species—from stable liquids like water and methyl formate to fleeting intermediates like methylene.